potassium valency|what is the valence electron of potassium : iloilo How many valence electrons are in one atom of each element? sulfur; helium; potassium; aluminum; Solution. Sulfur (S) is located in Group VIA (Group 16), .
McAfee Total Protection Protection for your devices with identity monitoring and VPN. Device Protection. Antivirus Virtual Private Network (VPN) Mobile Security Free Tools & Downloads . Corporate Headquarters 6220 America Center Drive San Jose, CA 95002 USA. Products. McAfee+Land Transportation Office or known as LTO is a government agency in the Philippines that are in charge for all land transportation in the country under the Department of Transportation. The Land Transportation Office or LTO role, is to enforce land transportation rule and regulations, traffic adjudication, issuance of permit and licenses .
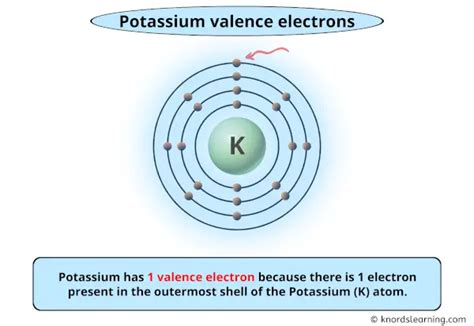
potassium valency,Potassium has a valence of +1, meaning it can form compounds with one electron donor or acceptor. This web page provides a table of element valences for all elements in the periodic table, with sources and definitions.
Valency of Argon: 18: 0: Valency of Potassium (K) 19: 1: Valency of Calcium: 20: 2: Valency of Scandium: 21: 3: Valency of .what is the valence electron of potassium Like most of the alkali metals, potassium compounds impart a characteristic color to flames. In the case of the 19th element, the color is pale lavender. Like sodium . Learn how to calculate the valence electrons of potassium (K) by using its electron configuration and the Bohr principle. Find out the valency, valence shell, and valence electron of potassium and how it .potassium valency what is the valence electron of potassium Mar 23, 2023
How many valence electrons are in one atom of each element? sulfur; helium; potassium; aluminum; Solution. Sulfur (S) is located in Group VIA (Group 16), .Element Potassium (K), Group 1, Atomic Number 19, s-block, Mass 39.098. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main . Note the valence electrons are in red. Helium has one shell filled by two red dots. Chlorine has 2 red dots on its inner most shell, followed by 8 red dots on the next .
Determine valence electrons using the periodic table. Learn how to determine the number of valence electrons for an element using the periodic table. An atom's valence electrons . Valence Definition in Chemistry. Valence describes how easily an atom or radical can combine with other chemical species. This is determined based on the number of electrons that would be added, lost, .An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different elements have. Fortunately, we can .
Potassium is a chemical element; . In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, which is easily removed to create an ion with a positive .
Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons .. Solution. a) Aluminum is in group III and so rule 1 predicts a valence of 3. Chlorine is in group VII and is not combined with oxygen or fluorine, and so its valence is 8 – 7 = 1 by rule 2. Each aluminum has three valence sites, while each chlorine has only one, and so it requires three chlorine atoms to satisfy one aluminum, and the formula .

Potassium Properties: Potassium's melting point is 63.25°C, boiling point is 760°C, specific gravity is 0.862 (20°C), with a valence of 1. Potassium is one of the most reactive and electropositive of metals. The only metal that is lighter than potassium is lithium. The silvery white metal is soft (easily cut with a knife).
potassium valency Potassium Properties: Potassium's melting point is 63.25°C, boiling point is 760°C, specific gravity is 0.862 (20°C), with a valence of 1. Potassium is one of the most reactive and electropositive of metals. The only metal that is lighter than potassium is lithium. The silvery white metal is soft (easily cut with a knife). Potassium is a vital element found in nature that plays an important function in a variety of biological activities. The valency of an element, or its combining power, is a basic notion in understanding its chemical properties and reactions. Let’s look into potassium valency using these crucial points: Definition and Importance:The valency of an element is a term that can be used to describe the affinity it will have to other atoms in the formation of chemical bonds. Another way of defining valency is to look at it as the number of available electrons for bonding in the outermost shell. The valency of some individual elements can be determined from their location on . Figure 5.2.1 5.2. 1 The shell structure of atoms of He, Cl, and K, as suggested by Lewis. Note the valence electrons are in red. Helium has one shell filled by two red dots. Chlorine has 2 red dots on its inner most shell, followed by 8 red dots on the next shell. The outer most shell has 7 red dots. The electron configuration of potassium is [ Ar] 4s 1. In the above electron configuration, the highest energy level (4) is marked with green color. The 4 th energy level contains 4s subshell and it has 1 electron. So potassium has a total of 1 valence electron. Next: Calcium valence electrons. Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition .
In general, the number of valence electrons is the same within a column and increases from left to right within a row. Group 1 elements have just one valence electron and group 18 elements have eight, except for helium, which has only two electrons total. Thus, group number is a good predictor of how reactive each element will be:

In chemistry, the valence (US spelling) or valency (British spelling) of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules.Valence is generally understood to be the number of chemical bonds that each atom of a given chemical element typically forms. Double bonds are considered to be two . There are two ways to find the number of valence electrons in Potassium (K). The first is to use the Periodic Table to figure out how many electrons Potassiu.
Potassium is the 19th element in the periodic table and has a symbol of K and atomic number of 19. It has an atomic weight of 39.0983 and a mass number of 39. Potassium has nineteen protons and twenty neutrons in its nucleus, and nineteen electrons in four shells. It is located in group one, period four and block s of the periodic table.In tertiary amines the valency of nitrogen atom is View Solution In moon rock sample the ratio of the number of stable argon- 40 atoms present to the number of radioactive potassium − 40 atoms is 7 : 1 .
Method 1: From the Periodic Table. To find out the valence electrons of Potassium, you have to see the position of potassium in the periodic table. More specifically, you have to see the group wise position of Potassium element in the periodic table. From the above image, you can see that Potassium (K) is present in the group 1 .
The valency or valency chart is helpful in order to determine how many atoms of an element will combine with another element to form any chemical formula. Another important use of valency of elements is to find or deduce formulae of compounds. If we know the valency of elements, then we can easily write formulae of compounds of .
potassium valency|what is the valence electron of potassium
PH0 · zinc valency
PH1 · what is the valency of oxygen
PH2 · what is the valence electron of potassium
PH3 · valency table class 10
PH4 · valency class 9
PH5 · silicon valency
PH6 · all elements valency
PH7 · Iba pa
PH8 · 1 to 20 valency